Clinical Relevance of the Sensorimotor Pathways
in Dysphagia Management following Tracheostomy
Kimberly Morris, MS, CCC-SLP, BCS-S, IBCLC
The oropharyngeal and esophageal swallowing systems are a challenge to understand fully due to the interdependence of sensory, motor, and behavioral systems. When patients are tracheostomy-dependent, assessment of their swallowing, establishment of the least restrictive diet, and identification of interventions to improve swallowing function pose a more difficult challenge than with patients who have an intact system.
Airway protection in these patients becomes highly dependent on reintegration of the upper aerodigestive tract, use of compensatory abilities, medical status, and the integrity of the physiologic aspects of swallowing. An understanding of these systems is an essential precursor to appreciating how they interplay and relate to swallowing safety in patients with tracheostomies.
Sensory inputs in the oral cavity contribute to the efficient preparation and transport of a bolus (bite of food or liquid), while sensory receptors in the pharynx facilitate timely initiation of the swallow (Sinclair, 1970). Aberrant sensations within the areas of the upper aerodigestive tract may have various negative effects on the oropharyngeal swallowing processing. The sensory innervations that are critical to the swallow process include the maxillary branch of the trigeminal nerve (V2), the mandibular branch of the trigeminal nerve (V3), the facial nerve (VII), the glossopharyngeal nerve (IX), as well as the superior laryngeal and recurrent laryngeal branches of the vagus nerve (X SLN/RLN) (Jafari, Prince, Kim, & Paydarfar, 2003).
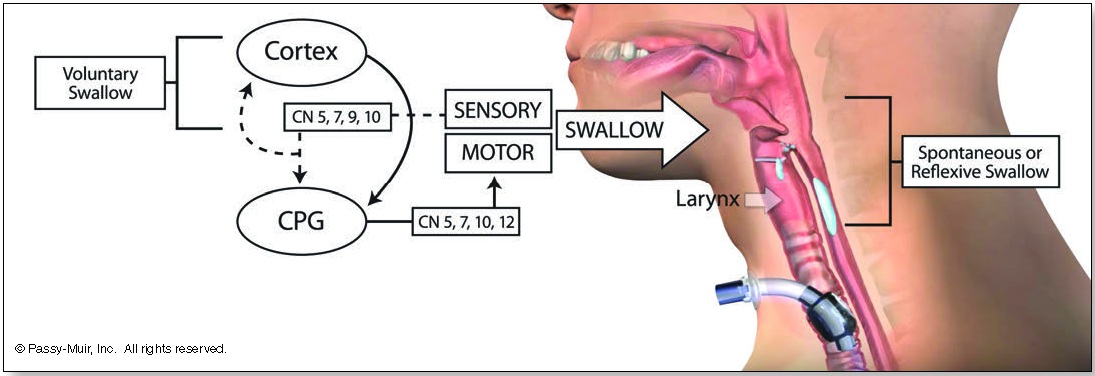
The primary contributors for motor innervation of swallowing include the mandibular branch of the trigeminal nerve (V3), the facial nerve (VII), the vagus nerve (X), the hypoglossal nerve (XII), and the ansa cervicalis (C1-C2; XII). Although the cranial nerves (CN) receive much attention when evaluating the stability of an individual’s swallowing potential, both cortical and subcortical structures are involved. Sensory input by way of afferent (sensory) pathways carries vital information to the swallowing centers of the brain. This swallowing center, known as a central pattern generator (CPG), has effects on swallowing, such as triggering swallow initiation, shaping the swallow, and timing the sequence of the swallow (see Figure 1). The motor activity is flexible and dependent on the response to sensory input. This relayed information, such as bolus size (size of the bite) and location, is vital to the performance of the swallowing process.
Bidirectional sensory afferent (superior laryngeal nerve) and motor efferent (recurrent laryngeal nerve) input to the central nervous system in response to stimulation of laryngopharyngeal mucosa typically results in firing of the thyroarytenoid muscle bilaterally in order to close the vocal folds (adduction) for airway protection (Domer, Kuhn, & Belafsky, 2013). More complex in nature is the swallowing process, which can be modulated both volitionally and reflexively, through sensory input (Steele & Miller, 2010). The input provided to the sensory system for elicitation of a functional motor response can be affected when airflow through the upper aerodigestive tract is bypassed with a tracheostomy. For example, bypassing the supraglottis (above the vocal folds) and subglottis (below the vocal folds) may compromise the laryngeal adductor response (Martin, et al., 1999)
The Passy Muir® Valve (PMV) is a unique option for patients with tracheostomies. The PMV may be used with a tracheostomy to restore a closed system. The Valve functions by closing at the end of inspiration and redirecting airflow during exhalation through the upper airway and out of the mouth and nose. This redirection of airflow would allow for increased distribution of sensory input to the larynx, pharynx, and oral cavity through re-establishment of the closed system (e.g., no escape of airflow through the tracheostomy tube) during swallowing. This effectively re-establishes subglottic pressure to facilitate respiratory and swallow processing by re-integrating the upper airway into the processes. Closing the system with a PMV generates a significantly improved environment for reception of sensory information and an opportunity to maximize the physiologic swallowing potential.
However, airway protection and overall swallowing function are not solely dependent on re-establishment of a closed system nor does a closed system dictate if a patient is safe to eat. As stated by Jadcherla (2017), “pharyngeal or esophageal stimulus evokes regional (pharyngo-esophageal reflex responses within the upper digestive tract), extraregional (responses within pulmonary and cardiac systems), and neurocognitive (sensation, perception, regulation of integrative reflexes) responses” (p. 14).
Further, a patient’s control of respiration and the effect that swallowing may have on respiration and laryngeal responses must also be considered. The specific types of respiratory control and swallowing effectiveness for a patient depend on the consistency of the food and liquids being consumed, the manner in which someone receives oral intake, as well as the age of the patient, initial indication for tracheostomy, and other comorbidities. For example, establishment and maintenance of airway closure is different for single, large bolus swallows as compared to small, sequential swallows from a bottle (Gokyigit et al., 2009; Lazarus et al., 1993). Infants who are breastfeeding have even greater differences in swallow-respiration patterns than seen with bottle feeding.
Single sips of a liquid may serve as a reasonable means to maintain nutrition for an older child or adult who cannot sustain airway closure across sequential swallows. However, the integrity of an infant’s laryngeal function and individual central pattern generators for respiration, sucking, and swallowing are essential in determining airway safety when feeding. Establishing an optimally closed system (e.g., through use of a Passy Muir Valve) during swallowing allows for generation of the subglottic pressure needed to establish and maintain laryngeal elevation with approximation of the arytenoids to the lower epiglottic petiole. This pattern may aid in more timely initiation of swallowing, improved airway protection, and more efficient respiratory processes.
Given that infants are also at risk for reduced minute ventilation when feeding, efficient swallow-respiration patterns are essential elements to consider when attempting to optimize respiratory reserves and potential for oral intake. Assessment of baseline respiratory dynamics and any changes to both swallowing pattern and safety in various feeding contexts are needed to determine if airway protection is being achieved and maintained across a feeding. For example, when evaluating an infant, several conditions should be assessed. During the assessment, the clinician should consider unpaced bottle feeding, paced bottle feeding, and feeding with AND without the Passy Muir Valve. These considerations are combined with different nipples that may change the suck:swallow:breath ratio and provide valuable insight into the safest avenue for feeding.
Although the sensorimotor benefits of accessing the upper airway and generation of ideal subglottic pressures during swallow are more easily achieved when using the Passy Muir Valve, this does not always equate to airway safety. Special consideration in assessment of breastfed and bottle-fed infants who depend on rapid and timely sequencing of airway closure when eating must be investigated. A patient’s compensatory strategies to meet respiratory needs may be uniquely demonstrated during feeding. For example, in babies with reduced respiratory efficiency that is not due to laryngeal abnormalities (e.g., diaphragmatic paresis, Spinal Muscular Atrophy), their systems may be hypersensitive to the slight increase in time for the expiratory phase to be completed.
While Passy Muir Valve use during feeding assists with closing the system and restoring pressures and sensation, use during non-feeding tasks also may be essential due to providing increased PEEP (positive end-expiratory pressure), sensation, increased swallowing frequency for secretion management, sustained voicing, and auditory self-feedback for speech/language development. The benefits achieved with a closed system are not limited to feeding only.
However, it is important to note that in children with tracheostomies the presence of the tracheostomy tube does not dictate that aspiration and impaired swallowing function will occur. The ability of a patient to achieve the necessary sensorimotor processes for airway protection are paramount, and the direct benefits that a Passy Muir Valve has on airway safety during breast/bottle feeding or cup drinking depends on the overall stability of the child’s system. Knowing the stability of each system (motor and sensory) is essential in treatment planning and generating the least restrictive plan. Assessment of swallowing function, with and without Valve placement, should always be assessed during bedside evaluations, Flexible Endoscopic Evaluations of Swallowing (FEES), and Modified Barium Swallow Studies (MBSS). Valve use may address conditions that would otherwise make risks of aspiration higher. During these assessments, attention to oropharyngeal swallowing functions that support airway protection or result in compromise is critical.
If physiologic function and airway protection appear similar, the benefits of using the Passy Muir Valve during meals to optimize sensory feedback from the larynx and the potential for improved cough to expel aspirated material should be strongly considered when making follow-up recommendations. In addition, if a patient is not able to restore upper airway access through Passy Muir Valve use, the medical team should assess tracheostomy tube size and consider direct visualization of the suprastomal trachea. In some circumstances, use of the PMV is unable to be achieved due to limitations with airway patency. Although reassessment of candidacy should be ongoing, ways to improve access to the upper aerodigestive tract should be explored to allow for sensorimotor integration in support of feeding progression. Improving access may include continued trials in therapy or simply short bursts of tracheostomy tube occlusion on exhalation to redirect airflow through the upper airway. During treatment, observation of the effects on swallowing frequency, swallowing recruitment, physiologic stability, sensory response to pressure changes, vocal function, and oropharyngeal clearance should be observed.
Oropharyngeal swallowing function is dynamic in adults and pediatrics with comorbid diagnoses, especially in those who are tracheostomy dependent. Determination of airway safety when feeding and establishment of interventions to improve swallowing function are dependent upon the stability and integrity of individual systems. Additionally, the modulation of the central nervous system with the medullary controlled swallowing centers include significant contributions from both sensory and motor pathways (Lowell et al., 2008; Ludlow, 2015). Therefore, the use of a Passy Muir Valve to re-establish a patient’s access and utilization of the upper aerodigestive properties and functions, including voice production, has the potential to optimize swallowing safety and function. However, swallow processing under certain contexts (e.g. high respiratory demands, conditioned behavioral responses, altered laryngeal/pharyngeal anatomy) is highly variable and individual compensatory differences may exist. Thorough assessment of swallowing physiology and generation of specific therapy targets should be completed in more than one context. This includes swallowing assessment with the use of a Passy Muir Valve, as well as with an open tracheostomy tube, in order to identify unique characteristics of each swallowing system that affect overall safety and progression of skills. This complete assessment allows for the best possible outcomes.
This article is from the Fall 2019 Protocol Issue of Aerodigestive Health. Click here to view Clinical Relevance of the Sensorimotor Pathways in Dysphagia Management following Tracheostomy.
References:
Domer, A. S., Kuhn, M. A., & Belafsky, P. C. (2013). Neurophysiology and clinical implications of the laryngeal adductor reflex. Current Otorhinolaryngology Reports, 1(3), 178–182. doi:10.1007/s40136-013-0018-5
Gokyigit, M. C., Pazarci, N. K., Ercan, I., Seker, S., Turgut, S., & Ertekin, C. (2009).
Identification of distinct swallowing patterns for different bolus volumes. Clinical Neurophysiology, 120(9), 1750–1754. doi: 10.1016/j.clinph.2009.07.039 Jadcherla, S. R. (2017). Advances with neonatal aerodigestive science in the pursuit of safe swallowing in infants: Invited Review. Dysphagia, 32(1), 15–26.
doi: 10.1007/s00455-016-9773-z
Jafari, S., Prince, R. A., Kim, D. Y., & Paydarfar, D. (2003). Sensory regulation of swallowing and airway protection: A role for the internal superior laryngeal nerve in humans. The Journal of Physiology, 550(1), 287–304. doi: 10.1113/ jphysiol.2003.039966
Lazarus, C. L., Logemann, J. A., Rademaker, A. W., Kahrilas, P. J., Pajak, T., Lazar, R., & Halper, A. (1993). Effects of bolus volume, viscosity, and repeated swallows in nonstroke subjects and stroke patients. Archives of Physical Medicine and Rehabilitation, 74(10), 1066–1070. doi: 10.1016/0003-9993(93)90063-g Lowell, S. Y., Poletto, C. J., Knorr-Chung, B. R., Reynolds, R. C., Simonyan, K., & Ludlow, C. L. (2008). Sensory stimulation activates both motor and sensory components of the swallowing system. NeuroImage, 42(1), 285–295.
doi: 10.1016/j.neuroimage.2008.04.234
Ludlow, C. L. (2015). Central nervous system control of voice and swallowing.
Journal of Clinical Neurophysiology, 32(4), 294–303. doi:10.1097wnp.0000 000000000186
Martin, J. H., Thomson, J. E., Aviv, J. E., Kim, T., Diamond, B., Sacco, R. L., & Close, L. G. (1999). Laryngopharyngeal sensory discrimination testing and the laryngeal adductor reflex. Annals of Otology, Rhinology & Laryngology, 108(8), 725–730. doi: 10.1177/000348949910800802
Sinclair, W. (1970). Initiation of reflex swallowing from the naso- and oropharynx.
American Journal of Physiology-Legacy Content, 218(4), 956–960. doi: 10.1152/ ajplegacy.1970.218.4.956
Steele, C. M., & Miller, A. J. (2010). Sensory input pathways and mechanisms in swallowing: A review. Dysphagia, 25(4), 323–333. doi:10.1007/s00455-010-9301-5
Article Summary
Kristin King, PhD, CCC-SLP
Use of a Speaking Valve in Children
Zabih, W., Holler, T., Syed, F., Russell, L, Allegro, J. & Amin, R. (2017). The use of speaking valve in children with tracheostomy
tubes: What is the scope of the literature. Respiratory Care, 62(12):1594-1601. doi: 10.4187/respcare.05599
This article provides a scoping review of the research related to children with tracheostomy tubes. From their review, the authors synthesize and summarize the current evidence on the use of one-way tracheostomy tube speaking valves in the pediatric population. From their initial search, the authors identified a total of 524 articles. After using inclusion and exclusion criteria, a total of 12 articles met inclusion criteria. The authors identified the levels of evidence (using the Sackett levels of evidence) to evaluate the qualitative strength of the evidence provided by the 12 studies and found that six studies were level 5, four were level 4, and two studies were categorized as level 3 evidence. The authors found that eligibility criteria for trials of speaking valves were inconsistent across all studies. The authors shared that all included studies had been conducted with the Passy Muir Valve®.
Much of the reviewed literature focused on tolerance or successful use of speaking valves in children with a tracheostomy but provided limited evidence on its impact on verbal communication. Four studies addressed successful use of the speaking valve as a primary focus and all studies reported use without adverse events during wake hours in 100% of the participants, and one study reported similar findings when used during sleep. Another benefit found in pediatrics was verbalizations and communication attempts. Various parameters for speech were assessed, including modal voice, phonation type, pitch, loudness, breath support, and voice continuity. The studies recorded spontaneous speech in older children and babbling in infants and those in the prelinguistic developmental stage. Communication attempts and verbalizations were found to be feasible in 74.3% of the children on first use. Additional benefits related to secretion management, improved cough, improved swallowing, ease of breathing, and reduced aspiration were a secondary focus in 50% of the studies. Current evidence on the use of the Passy Muir Valve in children with a tracheostomy demonstrated multiple benefits for infants through older children.











